
New confocal microscope is a ‘future-proof’ game-changer
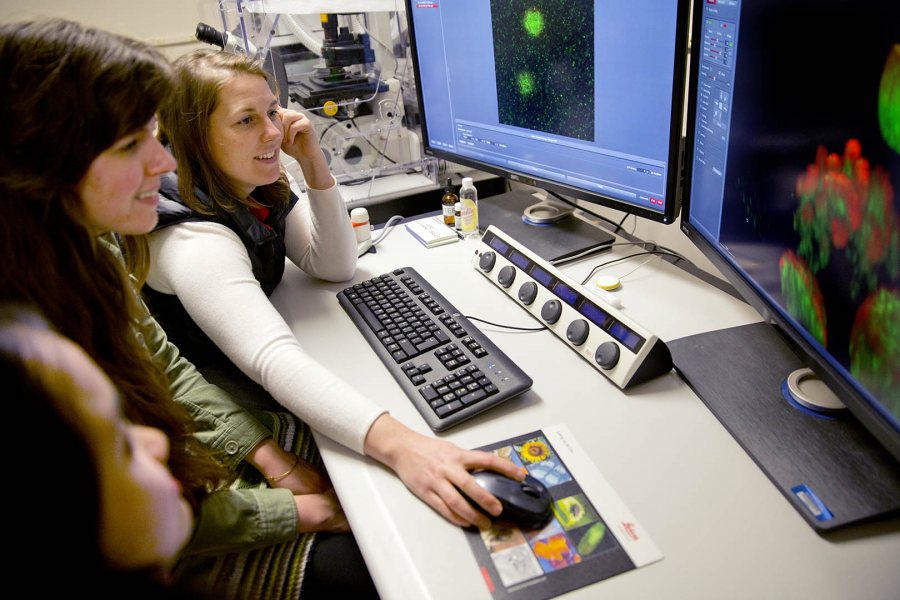
From lower left, seniors Minh-Tam Pham and Kathleen Morrill look on as their adviser, biology professor Larissa Williams, scans protein molecules with Bates’ new confocal microscope. (Phyllis Graber Jensen/Bates College)
Bates College is now home to a state-of-the-art microscope, only the second of its kind in the state, that professors call a “game-changer” for student learning and faculty research.
Called a “confocal” microscope because of the optical technology it employs, the new Leica SP8 is a versatile, user-friendly device that gives Bates a variety of important new or improved imaging capabilities. Bates was able to obtain the microscope through a grant awarded by the National Science Foundation.
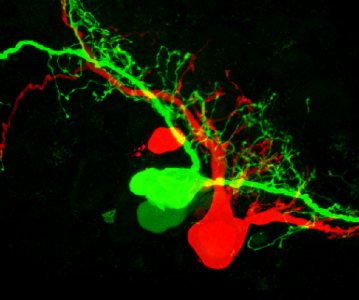
Taken with a confocal microscope, this image shows two adjacent neurons filled red and green fluorescent dye. The image, says Bates neuroscientist Nancy Kleckner, can tell researchers if the cells are coupled, “that is, whether the dye could leak from one cell to the other.” These types of neurons are involved in regulating feeding in pond snails, a specific focus of Kleckner’s research.
The microscope can create three-dimensional internal images of transparent specimens. In addition, unlike some other high-resolution imaging technologies, confocal microscopy requires only minimal, non-destructive specimen preparation — meaning, for instance, that biologists can use it to examine structures and processes within living cells.
The new scope will support research in disciplines including biology, neuroscience, nanotechnology and photophysics.
The Bates scientists awarded the grant are Matthew Cote, associate professor of chemistry; Travis Gould, assistant professor of physics; Nancy Kleckner, associate professor of biology; and Larissa Williams, assistant professor of biology and the leader of the application and acquisition process.
The NSF awarded Bates the $791,480 grant in September, and the German-built Leica was installed in a microscopy suite in the college’s Carnegie Science Hall in November. The grant was approved on the applicants’ first try. There are other confocal microscopes in Maine, but “the only comparable microscope to the Leica is at The Jackson Laboratory, in Bar Harbor,” said Joseph Tomaras, associate director of Bates’ office for external grants.

Biology professor Larissa Williams manipulates the specimen stage of Bates’ Leica SP8 confocal microscope. (Phyllis Graber Jensen/Bates College)
If the Bates team is elated by the Leica’s features and research potential, Williams explains that in terms of Bates’ teaching capacity, the new scope is a game-changer. “It’s incredibly important that students have access to this state-of-art technology,” she says.
“This microscope is future-proof.”
Confocal microscopy uses lasers, computers and optical elements to compose a complete image one pixel at a time. Key to this process is a version of the art-photography student’s old friend, the pinhole, which helps remove unwanted information from the image. While the basic principles of the system date back nearly 60 years, it took decades for technology to catch up with theory.
These microscopes create optical sections — the rendering of images in thin uniform layers that can be digitally stacked into a three-dimensional representation. For Williams and Kleckner, this ability to look inside cells is key.
Kleckner’s plans for the scope include research into the nervous systems of pond snails and zebrafish. Williams, who studies the effects of environmental toxicants on animal development, also works with zebrafish, but in the embryonic stage.
Both rely on so-called fluorescence microscopy, in which certain wavelengths of light are used to “excite” — or cause to glow — biological specimens that have been treated with fluorescent dye or genetically modified to light up. Until now — barring a road trip to use confocal scopes in Bar Harbor or at Bowdoin College — their go-to imaging tools were widefield scopes that can’t create optical sections.
The SP8 is a big step forward. “It allows us to make crisp, well-resolved images of deep cellular structures that our older microscopes would show as fuzzy,” says Williams.
For Gould, who specializes in nanoscopy, the Leica’s user-friendliness is an asset when he needs to test results from an experimental microscope that he or a student is working on. “You can just throw your sample on there, and see what you’ve got, in a quick and easy interface.” He’s also intrigued by the SP8’s ability to analyze the workings of fluorescent particles over a period of time within a sample.

The confocal workstation. The Leica microscope itself is to the left of the monitors. (Phyllis Graber Jensen/Bates College)
And the new scope’s ability to produce finely tuned wavelengths of laser light to illuminate samples is an advantage across the board.
Cote’s research is in nanotechnology, and the more precisely he can control the color of light that he trains onto nanostructures, the better he can understand their distinctive features.
“The bottom line is that, I think, Leica really has the best system out there, hands down,” says Gould. “It’s extremely flexible, extremely powerful, extremely easy to use. We’re very fortunate that we were able to get a system like this.
“And very fortunate that we got the grant for it on the first try, which is usually not the case.”
The confocal scope shares Bates’ microscopy suite with a JEOL scanning electron microscope that the college purchased in 2012. The two instruments are complementary. The SEM can depict smaller objects than the confocal, Cote explains, and is also equipped to chemically identify elements in a specimen. But preparing specimens for it can be destructive to them.
The confocal microscope provides non-destructive, true three-dimensional imaging, even of internal cellular structures and even of live cells. In fact, says Gould, “With the confocal you can target specific biomolecules for imaging, which is an important advantage over electron microscopy.”
“Because of the SP8’s capabilities, researchers at Bowdoin wrote to the NSF in support of our proposal,” Tomaras said. He added that the microscope will be incorporated into professional training in advanced imaging for faculty and students at Southern Maine Community College.






