Chemical Safety
Jonathan Witt is the college’s Chemical Hygiene Officer. Please reach out to him if you have any questions.
This web based summary of Bates College’s Chemical Hygiene Plan (CHP) is intended to make chemical safety information readily available. Use the tabs below to explore topics related to chemical safety. The unabridged Chemical Hygiene Plan can be accessed here.
+Hazardous Chemical Definition
“A chemical for which there is statistically significant evidence based on at least one study conducted in accordance with established scientific principles that acute or chronic health effects may occur in exposed employees. The term “health hazard” includes chemicals which are carcinogens, toxic or highly toxic agents, reproductive toxins, irritants, corrosives, sensitizers, hepatotoxins, nephrotoxins, neurotoxins, agents which act on the hematopoietic systems, and agents which damage the lungs, skin, eyes, or mucous membranes.” OSHA Lab Standard (29 CFR 1910.1450)
+Chemical Labels and Signs
The United Nations has established a Globally Harmonized Standard (GHS) for the classification and communication of chemical hazards. More recently the OSHA Hazard Communication Standard has been changed to be compatible with the GHS. Therefore, a single unified standard exists that encompasses visual and written descriptions of chemical hazards.
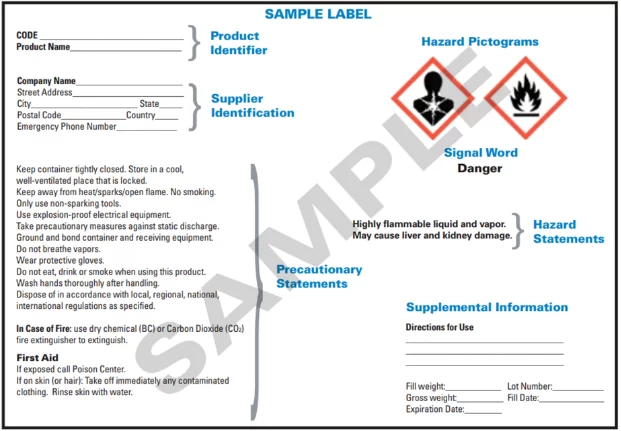
Primary Container Labels
Any chemical container that is purchased from a chemical vendor is required to have a label that contains the following information:
- Product identifier (CAS Number/Name)
- Signal Word
- Hazard Statements
- Pictograms
- Precautionary Statements
- Information about the manufacturer
The following is an OSHA example of a label that meets the unified GHS/Hazard Communication Standard.
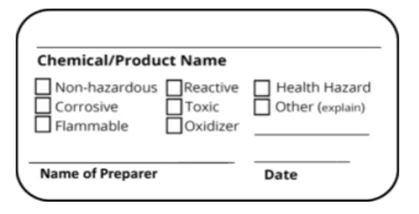
Secondary Container Labels
Secondary or “workplace” containers are often used in the laboratory to hold smaller amounts of pure chemicals, mixtures or solutions. There is more flexibility concerning how these containers need to be labeled. OSHA requires that secondary containers identify the chemical or solution and communicate the hazards associated with it. The identification does not have to follow GHS standards for original chemical containers. It can consist of words, pictures or symbols that effectively communicate the chemical hazards.
The following is an example of a label that would satisfy these requirements:
Door Signs
Door signs are a way to communicate the hazards in a particular lab. Each research lab should have a door card that specifies chemical, biohazard and radiation hazards. These cards also indicate the level of PPE that is required in a particular lab. This is an example of a door card.
+Safety Data Sheets (SDS)
Safety Data Sheets (SDS) are documents provided by a chemical manufacturer or vendor that describe the hazards associated with a particular substance. SDS follow a set format adopted with the Globally Harmonized Standard. SDS contain important information such as health and physical hazards, composition, reactivity and toxicity. These documents are intended to provide hazard identification and awareness for laboratory workers or first responders to a chemical involved incident.
There are a number of ways that you can access an SDS. You can access SDS for chemicals in our inventory by logging into Chemwatch (see SDS instruction doc). SDS can also be found on the vendor’s website.
+General Laboratory Safety Rules
- Do not obstruct access to exits or to safety equipment (spill kits, fire extinguishers etc.)
- Bench tops and hood areas should be free of clutter.
- Glassware should be washed promptly.
- Chemicals should be stored in an organized fashion, with the labels turned outwards so that they are visible.
- Bags, coats and other personal items should be stored outside of the lab.
- Secondary (non-vendor) containers need to be properly labeled. Please see section 3.1 for more detailed requirements.
- Keep containers away from the edge of the bench to reduce the possibility of an accident.
Food, drink, smoking and vaping are never allowed in the laboratory.
+Personal Protective Equipment
Eye Protection
Some form of eye protection is mandatory while in research labs when actively working with or near chemicals. Either safety glasses or goggles conforming to the ANSI Z87.1 standard should be used. Safety goggles should be worn when there is a risk of splashing chemicals or explosion because they provide a higher level of protection.
Gloves
Gloves should always be worn in the laboratory. However, they should never be worn outside the laboratory, while holding a phone or typing on a computer, or while interacting with anything that should not be contaminated with chemicals.
Gloves are intended to provide a layer of protection between chemicals and the hand. However, typical gloves used on a daily basis in chemistry labs are not designed to protect the user during continuous exposure. They are intended to be used to briefly protect the hand in case of accidental contact.
Gloves are made out of many different polymers. Each type works well depending upon the situation and chemicals being used. However, no glove material provides ideal protection against all chemicals. The two common types of gloves used in Bates chemical laboratories are:
- Nitrile gloves (at least 4 mils thick) are the most frequently used gloves at Bates. They provide some degree of protection against organic chemicals. These are appropriate for day-to-day experiments using organic or inorganic chemicals. Though they offer better protection than latex gloves to organic chemicals, many solvents will permeate the glove within seconds. Gloves only buy time.
- Latex gloves provide good protection when working with aqueous chemicals. They are less resistant to organic chemicals than nitrile gloves. Latex gloves are known to cause sometimes serious allergic reactions. Laboratory workers with known latex allergies should never use latex gloves.
Lab Coats
Lab coats are not mandatory in chemical laboratories at Bates. Some principal investigators choose to use them in their labs. It is important that the lab coat material is compatible with the work being performed. Many lab coats are made out of cotton or polycotton blends. These offer fair protection against chemical contact or splashes, providing another barrier between the hazard and the skin. However, they are known to be highly flammable. If an experiment requires the use of large amounts of flammable solvent or use of pyrophoric agents, a flame resistant lab coat should be used.
+Chemical Storage and Transport Guidelines
General Chemical Storage Guidelines
- A date should be recorded on the chemical container when it is first opened. This is the responsibility of the end user. In some cases, the date the container was opened is more significant than the date it was received (e.g. ethyl ether).
- Whenever possible, sturdy shelves with a solid surface and a lip should be used.
- Chemicals should not be stored above eye level whenever possible.
- Heavier items or larger liquid containers should be stored closer to the ground.
- Chemicals should be organized by storage groups to avoid mixing of incompatible chemicals. An explanation of storage groups is here (link).
- Chemicals can be organized alphabetically, but only if incompatible chemicals are stored separately.
- Containers should be inspected regularly. If a container is compromised (rusting, dented, bulging etc), the chemical should be disposed of as hazardous waste.
- Chemicals should not be left out for extended periods in hoods or on the benchtop.
- Chemicals should be returned to storage after each laboratory session. This decreases the likelihood of a spill.
Transport
The following practices should be observed when moving hazardous chemicals. Similar protocols apply to transporting chemicals within a building and transferring them between buildings.
- Anyone who transports chemicals must be familiar with the hazards of the substances they are moving. They also must know what to do in the event of a spill.
- Secondary containment is mandatory. For a single 1 liter or 4 liter bottle, an appropriately sized bottle carrier can be used. If more than one container is being transported, a cart and plastic bin should be used. The cart must have a 2 inch solid lip.
- A lab coat and goggles may be prudent.
- Gloves should never be used when transporting chemicals. This is the case even if your gloves are clean.
- The route should be planned ahead of time, considering any obstacles. Chemicals should be moved when there will be the least number of people in the hallways (during classes).
- Elevators should always be used for transporting chemicals.
- Smaller bottles can be transported in a zip lock bag.
- Gas cylinders must be moved using an appropriate hand cart designed for moving cylinders and which features a chain for securing the cylinder. Before moving a gas cylinder, whether or not it is empty, remove the regulator and replace it with a cylinder cap.
+Fume Hoods
Fume Hoods
Chemical fume hoods are the most important type of engineering control for hazardous gases and aerosols. They are typically used when dealing with volatile chemicals or chemicals with a strong odor. If there is a risk of ingestion by inhalation, a fume hood should be used. General rules for using a fume hood are:
- Chemical fume hood exhaust fans should be left on at all times.
- When working in a chemical fume hood, the sash should be kept as low as is practical. If using a horizontal sash, the opening should be kept as narrow as possible. This will maximize the efficiency of the fume hood.
- Chemicals should be kept at least 6 inches from the front of the hood. This is the minimum distance required to ensure no fumes will escape.
- Close and open the sash slowly. If the sash is opened too quickly, the motion can create turbulence that will interfere with the efficacy of the fume hood.
- Keep the fume hood clean and uncluttered.
- Keep the sash closed when the fume hood is not in use.
- If performing a reaction that requires cooling with water, make sure that the tubing is attached to the glass with copper wire. The flow of water should be as slow as possible.
Snorkel Exhaust
Snorkel exhausts provide ventilation for small point sources of fumes or other harmful substances. They are particularly useful for capturing contaminants in circumstances in which a fume hood cannot be used. For example, they can be used to capture fumes from a large piece of equipment that cannot be kept in a fume hood. In order to be effective, the snorkel must be within approximately 2 inches of the fume source.
+Particularly Hazardous Substances (PHS)
Certain substances are considered to be particularly hazardous and therefore require more rigorous control measures. There are three types of chemical that fall in this category. A PHS is classified as either a “select carcinogen”, “reproductive toxin”, or a substance with a “high degree of acute toxicity.” If any of these substances are being used in a lab, a “designated area” where this substance will be used must be specified.
Select Carcinogens
Select carcinogens are found in the following lists:
- OSHA list of regulated carcinogenic chemicals (29 CFR 1910 Subpart Z).
- List of substances “known to be a carcinogen” in the National Toxicology Program Report on Carcinogens. Chemicals that are on the “reasonably anticipated to be carcinogen” list may be considered select carcinogens if statistically significant tumor incidence has occurred in animal models.
- International Agency for Research on Cancer (IARC) Group 1 carcinogens. Group 2A or 2B carcinogens may also be considered select carcinogens if statistically significant tumor incidence has occurred in animal models.
Select carcinogens can usually be identified by the hazard identifications on a Safety Data Sheet (SDS). Section 2 of the SDS typically includes either H350: May Cause Cancer, or H351: Suspected of Causing Cancer.
Reproductive Toxins
Reproductive toxins are substances that affect reproductive capabilities. Specifically, they are chemicals that create chromosomal abnormalities, or teratogens which cause the malformation of fetuses. Unlike select carcinogens, there is no consistent standard for what chemicals qualify as reproductive toxins. Chemicals that are known or suspected of being reproductive toxins include organic solvents, lead, ethylene glycol ethers, carbon disulfide, and ethylene oxide.
High Degree of Acute Toxicity
Like reproductive toxins, there is no set definition of what constitutes a substance with high acute toxicity. A standard that is sometimes employed is based upon the median lethal dose (LD50) of a substance. A substance with an LD50 < 50 mg is typically considered to have high acute toxicity.
Designated Areas
Any area designated for use of a particularly hazardous substance requires the following:
- Clearly mark the designated area with appropriate signage. The area may be an entire laboratory, an identified area within a laboratory or an isolating device such as a glove box or fume hood. The area or device signs should read “DANGER (Specific Agent), Authorized Personnel Only”.
- Emergency response procedures specific to the hazardous substance must be posted near the site.
- Detection/monitoring equipment may be required in laboratories where highly toxic chemicals (especially poisonous gases) are used. If uncertain, contact the CHO/EC.
Containment Devices
When working with particularly hazardous substances in fume hoods and glove boxes:
- Exhaust air from ventilation systems in which work is performed with carcinogens, reproductive toxins and acutely toxic chemicals may require scrubbing before release to the atmosphere. OSHA Permissible Exposure Limits or other regulatory standards may not be exceeded.
- Ventilation efficiency and operational effectiveness of containment devices used to manipulate or contain hazardous substances must be evaluated regularly according to a schedule determined by the Laboratory Supervisor. Proper use, maintenance and hygiene will ensure maximum protection from this equipment.
- Compressed gas cylinders containing acutely toxic chemicals must be stored in ventilated gas cabinets.
PHS and Lab Specific SOPs
If a laboratory must work with a Particularly Hazardous Substance, the PI for that lab must develop a lab specific SOP. An outline for a lab specific SOP can be found in appendix C. Before work with a PHS is started, a copy of the lab specific SOP must be submitted to the CHO and the director of EHS.
+Emergency Procedures
Chemical Spills
One of the major hazards associated with chemicals is the risk of a spill. The correct response to a spill depends upon the nature of the chemical and the size of the spill. Small chemical spills can often be managed by the personnel in the lab, while larger spills may require outside assistance.
Small Chemical Spills
Small chemical spills (less than 1 gallon of liquid, 1 pound for solid) can frequently be managed by lab personnel. Each space that contains chemicals will have its own spill kit consisting of the following:
- (3) 3” x 42” Absorbent “Socks”
- (5) 15” x 20” Absorbent Pads
- Nitrile Gloves
- Splash Proof Goggles
- 2 lb Sodium Bicarbonate (Baking Soda)
- 2 lb Citric Acid
- Heavy Duty Plastic Bags
- Bucket
The general procedure is as follows:
- The laboratory supervisor and workers should determine the magnitude of the spill and any hazards related to it (fumes, fire, broken glass).
- In the case of volatile solvent, pay particular attention to the quantity of solvent that has evaporated. It may be hazardous to breathe the fumes from a solvent spill.
- If the PI or laboratory workers conclude that they can proceed safely with immediately available PPE, they should swiftly begin the cleanup. Lab workers should never attempt to clean up a spill of a substance they are unfamiliar or uncomfortable with.
- The spill should be contained using absorbent “socks” or other means to create a physical barrier around the spill.
- If the spill is a strong acid or base solution, a neutralizing chemical should be used. Sodium bicarbonate can be use to neutralize acid, while citric acid can be used to neutralize base.
- Adsorbent pads should be used to soak up the spilled liquid.
- The pads should be collected and deposited in the plastic bags provided.
- The plastic bags should be sealed in the provided bucket.
- The bucket or container should be treated as hazardous waste and immediately deposited in the MAA, following normal documentation procedures for hazardous waste.
Large Chemical Spills
Large spills (greater than 1 gallon for liquid, 1 pound for solid) should not be handled by lab personnel. This also applies to smaller spills of materials that are determined to be too toxic or otherwise hazardous to be handled safely. In this situation, the following emergency procedure should be followed.
- Quickly leave the area and close the door.
- Alert campus security (207-786-6111) and EHS (608-395-4775) immediately.
- Alert the CHO (207-786-6294).
Chemical Exposure and Related Injuries
General Guidelines
A range of accidents and injuries can occur while working with chemicals in the lab. These include chemical burns and irritation to the skin, eyes, and lungs. Safety equipment and procedures are in place to address each of these risk factors. General safety procedures include:
- All lab personnel should be informed prior to any experiment of the potential safety hazards associated with the chemicals being used. SDS are available to students, faculty and staff as needed. They should also be aware of what should occur if an accident happens (use of eye washes, showers etc).
- In the event of a spill that contaminates a member of the lab, the affected person should be treated immediately. Spill clean up can occur after the injury has been addressed.
- Small chemical exposures can frequently be addressed by lab personnel. A small exposure of the skin or eyes may resolve itself after flushing with water.
- If a large or severe chemical exposure occurs, Safety and Security and EHS should be notified immediately. If necessary contact 911.
Small Chemical Skin Burns
In the case of a small area of chemical exposure, whether to corrosive, irritating, sensitizing or otherwise harmful chemical, the affected area should be immediately flushed with water. The affected area should be held under a steady stream of water for a minimum of 15 minutes.
Lung Exposure
In the case of accidental exposure of the lung to a corrosive or irritating chemical, the first step is to immediately remove the person to the outside. Fresh air is sometimes sufficient to resolve the situation. If the exposure is severe, or if the individual is still affected after 15 minutes of fresh air, contact EHS and 911 immediately.
Eye Burns
In the event of chemical exposure of the eyes, the emergency eye wash should be used. There are several types of eye wash stations on campus. The type that are in place in the Bonney Science Center have these designs.
For the eyewash station on the left, pull the lever on the left to activate the water. For the eyewash station on the right, push the red vertical button. For either type:
- Hold your eyes open, or ask someone to help you with this.
- The eyes should be rinsed for 15 minutes.
- If the burn is severe or if the water is not helping, contact campus security and EHS. If necessary contact 911.
Large Skin Burns
If a large area of the skin or clothing comes into contact with a toxic or corrosive chemical, the safety shower must be used.
- Activate the shower either by pulling the lever if in Bonney Science Center, or the chain if elsewhere on campus.
- Remove any clothing that has been contaminated.
- Remain under the shower for at least 15 minutes to ensure the chemical has been sufficiently washed off.
- Contact campus security and EHS immediately in the event of a large chemical exposure. Contact 911 if necessary.
Hydrofluoric Acid Burns
Hydrofluoric Acid (HF) is a highly corrosive and toxic chemical. It can cause severe chemical burns and severe pain. Special first aid and emergency procedures must be used. HF not only causes severe skin corrosion, but readily enters the body creating systemic issues.
HF Skin Contact
- Immediately have someone contact 911 then campus security and EHS.
- Flush the area with large amounts of water.
- Remove contaminated clothing while continuing to flush. Flush with copious amounts of water for 5 minutes.
- Apply 2.5% calcium gluconate gel to the wound. Massage the gel into the affected area. Reapply gel every 15 minutes until medical help arrives.
- Depending upon the concentration of the HF solution, symptoms can be immediate or take hours to manifest. Regardless of the concentration, the exposed area should be treated immediately and thoroughly.
HF Eye Contact
- Immediately have someone contact 911 then campus security and EHS.
- Hold the eyelids open and use the eyewash station to flush the eyes thoroughly with water for 15 minutes.
- The victim should seek immediate medical help, ideally from an eye specialist.
- Ice can be applied to the eyes while awaiting medical treatment.
Inhalation
- Immediately have someone contact 911 then campus security and EHS.
- Move the victim to fresh air.
- Await medical help.
+Chemical Waste
Hazardous Chemical Waste
In 1976, the legislature passed the Resource Conservation and Recovery Act (RCRA). This law directed the EPA to establish a regulatory framework for the handling and ultimate disposal of hazardous chemicals. This framework, which tracks waste from the point of generation through transportation, treatment and disposal, is referred to as the “cradle to grave” system. Hazardous waste generators are classified according to the volume of waste that they generate. Bates College is a Large Quantity Generator (more than 100 kg per month). This means that we are required to adhere to the most rigorous standards.
There are two ways that a chemical can be determined to be hazardous within the RCRA framework.
- The chemical is a “listed” waste. This means that the chemical is found on either the P, U, F or K EPA lists.
- The chemical has one of the following characteristics:
- Ignitability (flash point below 60 °C)
- Corrosivity (pH<2 or pH>12)
- Reactivity (substance may react violently under normal conditions)
- Toxicity (substance that is toxic and has the ability to leach into the ground if deposited in a land fill)
More detail on hazardous waste identification can be found in the annual hazardous waste training. Hazardous waste accumulation and disposal are regulated at both the state and federal level. The Maine state laws that regulate hazardous waste can be found in CMR Chapters 850-857. Bates College accumulates waste in four Main Accumulation Areas (MAAs). They are located in Bonney Science Center, Carnegie Science Building, Dana Hall and Cutten Maintenance. These storage areas are maintained by the CHO and EHS.
Satellite Accumulation Areas (SAAs)
Lab spaces that generate hazardous chemical waste on an ongoing basis should make use of Satellite Accumulation Areas (SAAs). SAAs are allowed by the state in the interests of convenience. There are several important features of SAAs:
- They must be inspected once a week. This is the sole responsibility of the person in charge of the lab space.
- The date that a container starts accumulating hazardous waste must be clearly documented on an SAA log sheet. Do not ever put a date on the container unless you intend to bring it to the MAA within 72 hours.
- The container must have a “hazardous waste” sticker.
- The contents of the container must be recorded.
- Incompatible hazardous waste must be separated. For example, strong acids and bases should never be accumulated in the same container.
- Halogenated organic waste must be separated from general organic waste.
- Containers must be closed at all times unless waste is being added.
- When the container is full, it should be taken to the MAA in the same building. A sticker listing the contents should be applied to the container. A neon hazardous waste sticker with the date should be applied to the container. The date and pertinent information about the container should be recorded in the logbook.
If you think you may need an SAA, reach out to Jonathan (jwitt@bates.edu)
Treatment of Hazardous Waste
Generally speaking it is not permissible to treat or dilute hazardous waste on campus. Bates is not permitted as a hazardous waste treatment facility. Therefore, we are not allowed to treat or dilute chemicals in almost all cases. Examples of procedures that are not allowed:
- Quenching of organometallic reagents.
- Quenching of peroxides.
- Dilution of strong acid/strong base solutions to meet the drain disposal allowed pH.
- Dilution of ethanol solutions.
- Allowing the contents of a container to evaporate to eliminate the waste is not allowed.
Aqueous solutions up to 500 mL can be treated to adjust the pH for drain disposal. It is not permissible to either dilute or treat waste of a larger volume. This waste must be disposed of with the hazardous waste.