
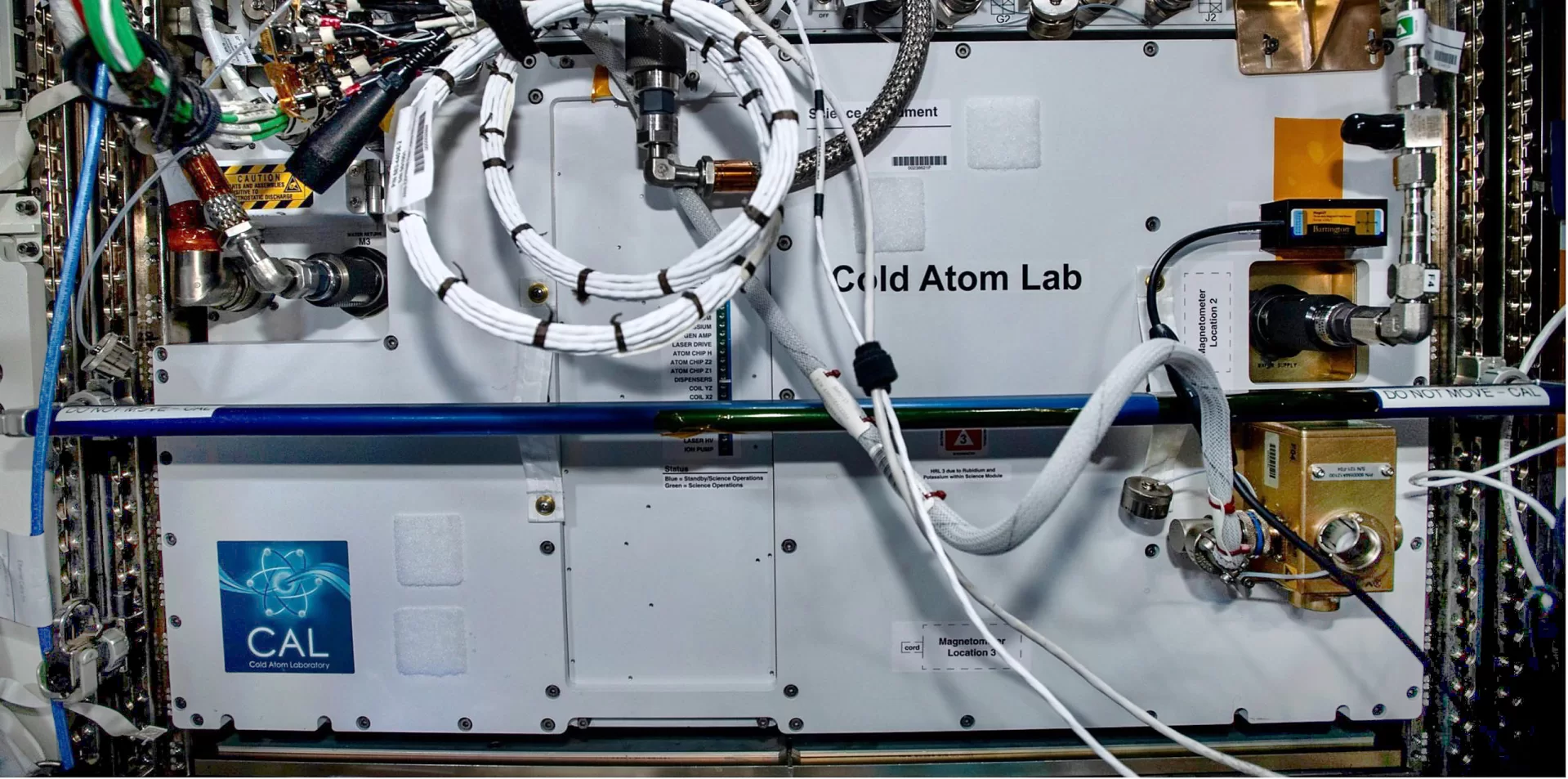
When your laboratory is a closed box, orbiting 250 miles above Earth on the International Space Station, it can be challenging to get a visual on what’s going inside the lab.
That was a problem that Professor of Physics Nathan Lundblad faced as the data from his experiments in the Cold Atom Laboratory started rolling in from the International Space Station a few years ago.
The equipment was working the way he’d planned when he conceived of the project and pitched it to NASA. Working with NASA to run the experiments remotely from Earth, he and his team had successfully created — for the first time ever — bubbles of ultracold atoms, which earlier physics had theorized could exist but which simply couldn’t be done on Earth, where the rules of gravity won’t allow it.

This huge advancement for science, and win for the Cold Atom Laboratory, known as CAL, was announced in 2022 and this spring led to two exciting pieces of news for Lundblad. The first was that NASA was extending his opportunities to do research in space with a $1.89 million award. Then the agency honored his breakthrough in manipulating atoms by giving him its NASA Exceptional Scientific Achievement Medal.
The space station’s program chief scientist Kirt Costello describes Lundblad’s work as “groundbreaking.”
“In the science program, we look for those people who have done investigations in the International Space Station and have also published findings that are really developmental for the field of science overall,” he says.
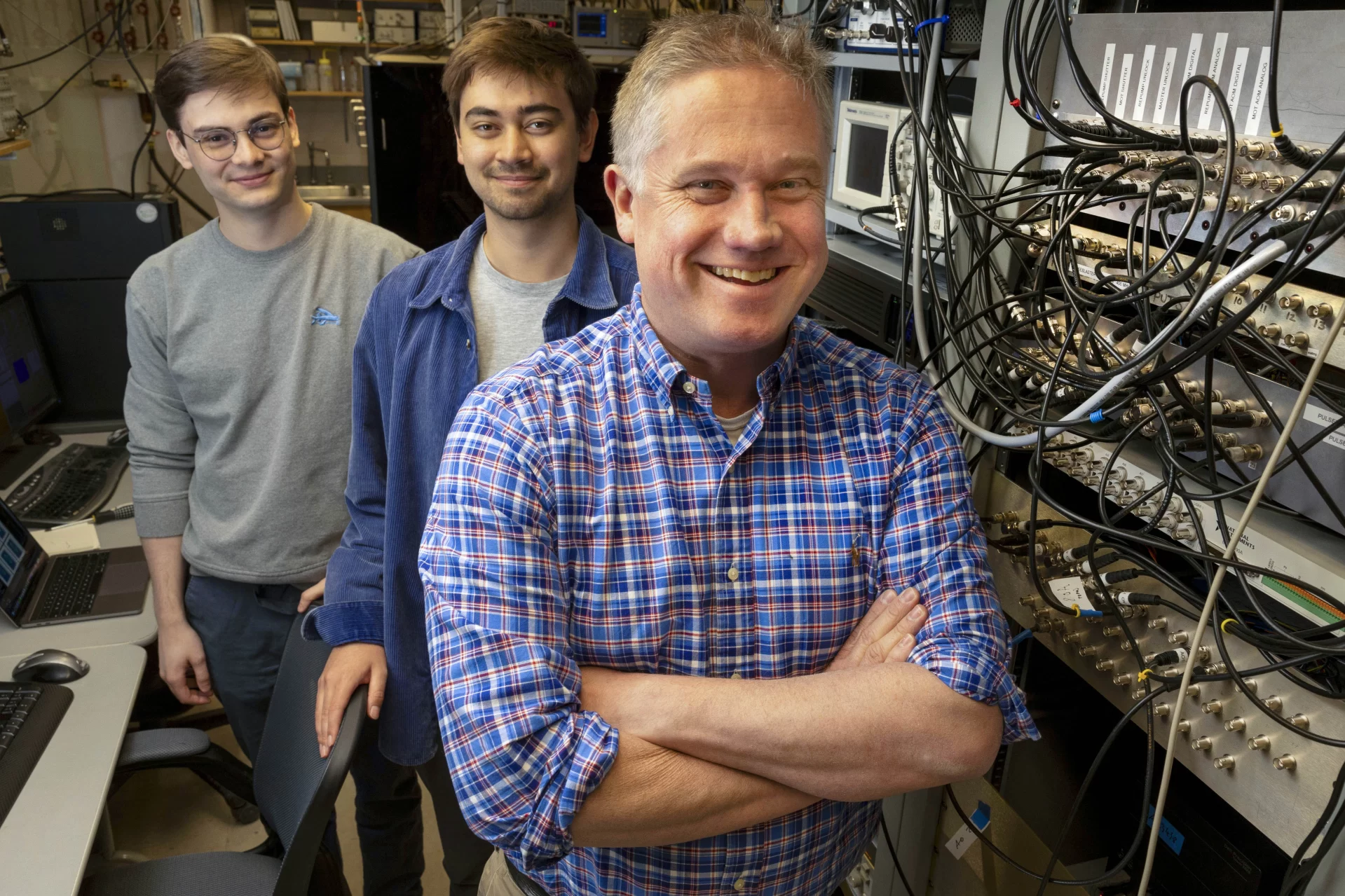
The new funding also meant that the assignment Lundblad had given one of the students who works with him in the Lundblad Laboratory at Bates was going to be more essential than ever. Earlier this academic year, he had asked Kona Lindsey ’23 of Colorado Springs, Colo., to develop software to make it easier to visualize what was happening with those experiments on the space station.
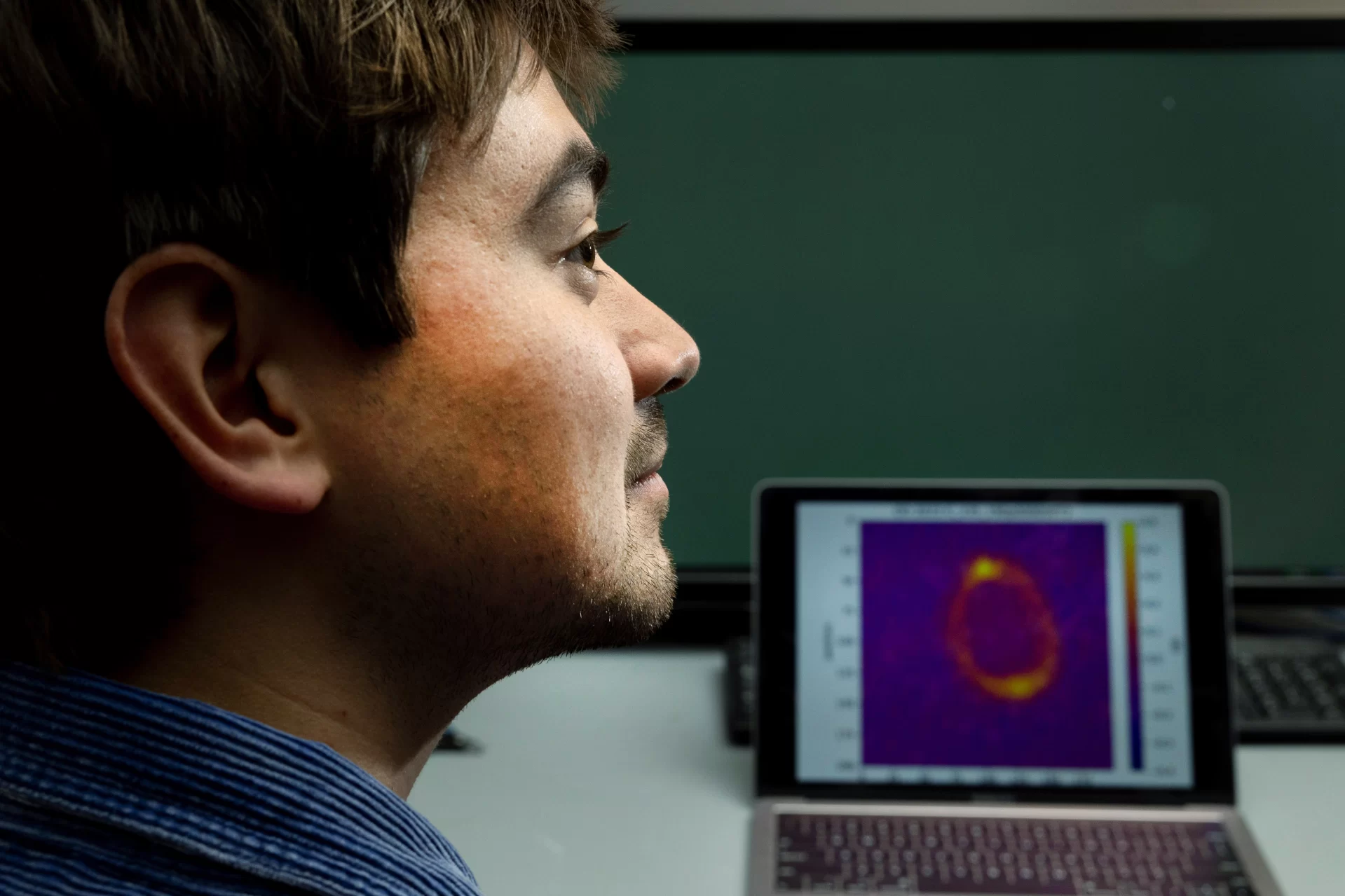
With the data Lundblad pulls in from this lab in the sky, literally hundreds of shots a day when it’s his turn on the shared lab, there’s a lot of analysis to be done. It’s not just about making the bubbles — or spheres — of atoms but understanding the topology of them, how they function and how they can be altered by changing elements of how they are created.
Lundblad’s “recipes,” tinkered with at extreme low temperatures in microgravity surroundings, include lasers, radio waves, rubidium, potassium, and various options for magnetic and optical trapping. Exploring these variables further is the plan. “But the first thing that needs to happen is, we actually need to sort of visualize it and say, ‘OK, let’s look at and analyze these clouds,’” Lundblad says.
“Historically, I sort of painstakingly loaded each image and pondered it and cycled through it all. But what I asked Kona to do is write a more coherent software package where I can just say, ‘Hey, what did yesterday’s data look like?’ And you can hit a few buttons and yesterday’s data just pops up on the screen nicely organized.”
Lindsey, he says, “exceeded my expectations” and established a baseline for future Bates students to build on. “Another student will continue this work for sure,” Lundblad says. “Because you can always make it better.”
If pressed, Lindsey, a physics and math double major with a minor in digital and computational studies, can boil it down to very simple terms, as he did when he was sharing his thesis at this year’s Mount David Summit: “There are pictures that are taken on the International Space Station of these experiments. And those pictures get sent back down to Earth. And I take those pictures and do some math on them.”
But the truth is, the work Lindsey is doing, along with the other physics majors Lundblad has brought into this project, involves complex science of mind-blowing proportions around what’s often described as a fifth state of matter (after solid, liquid, gas, plasma). Even Lindsey’s father, who teaches electrical engineering at the University of Colorado, finds what his son has been working with in the Lundblad Laboratory somewhat hard to grasp. “I think it’s a bit over his head,” Lindsey says with a smile.
The applications for this area of physics aren’t clear yet, but its scientific significance is. This work dates back to atomic behavior that Albert Einstein said in the 1920s he believed could happen — theoretically, at least — but doubted anyone would figure out a way to make it a reality. Those who succeeded some 70 years later became Nobel Prize winners, and today this area of science, revolving around something called the Bose-Einstein condensate, or BEC, is one of the hottest areas of physics research.
The name of the condensate honors the two geniuses behind the theory, Satyendra Nath Bose and Einstein. Bose was a young physicist from India who reached out to Einstein in 1924, sharing a statistical theory he’d developed about photons and asking for help getting it published. Einstein embraced it enthusiastically, made sure the theory was published and then continued building on Bose’s work.
What emerged was a quantum theory that individual atoms could turn into a superatom — many atoms behaving collectively as one — under very cold conditions. And not Maine winter cold, but rather, as close to absolute zero as possible. That’s the lowest theoretical temperature, which temperatures in space come closest to naturally.
But it took the better part of a century — 71 years — to make the first of these superatoms. Einstein probably wouldn’t have been surprised it took that long; when he described the state of matter that would become known as the Bose-Einstein condensate, he concluded with “this appears to be as good as it is impossible.” It’s no wonder the National Institute of Standards and Technology has referred to the quest to produce a BEC as a “Holy Grail.”

Adrian deCola ’23 of Southbury, Conn., a physics major with a minor in digital and computational studies, included a two-page summary of the history from idea to realization in his thesis. “I wanted a hook,” he says. DeCola worked in the Lundblad Lab in summer 2022, modeling and building a laser-driven cooling and purification apparatus for his professor. (Lundblad has two BEC machines in Carnegie, one he’s been running for about a decade and a newer one, under development, that’s meant to more closely mimic the one up in space.) DeCola included Einstein’s long-ago proclamation about what atoms could do, under the right circumstances, as well as that challenge he seemed to issue to future scientists by calling it “impossible.”
It’s been “super cool” to work on components of a scientific inquiry at this high level, deCola says, although he admits, he “had a ton of questions” initially. “I finally get it,” he says. “Sometimes it seemed like I was learning the point as I was doing it.”
Similarly, this was all new and intense material to Elias Veilleux ‘23 of Orono, Maine, when he started doing research with Lundblad in his sophomore year. But by the time he applied for a slot in Princeton University’s electrical engineering doctoral program last fall, he was well versed enough to describe his work in Lundblad’s older BEC machine in Carnegie to his interviewer. He landed a spot at Princeton, and is grateful for Lundblad’s guidance. “He’s been a really invaluable resource,” Veilleux said, including in helping him decide whether to pursue a master’s or a PhD., from both an intellectual and financial standpoint.
There are four other U.S. research groups sharing lab time with Lundblad’s team on the Cold Atom Laboratory. (The lab was devised to be something of a “jack of all trades” for scientists studying ultracold atoms, says Lundblad’s co-investigator Dave Aveline, who is based at the Jet Propulsion Laboratory in California.)
Several Nobel Prize winning physicists were involved in the planning stages of CAL, including Eric Cornell and Wolfgang Ketterle, who received their Nobel Prize in 2001 for making the first BECs in 1995, as well as William D. Phillips, who won the Nobel in 1997. When Cornell, Ketterle, and a third physicist, Carl Wieman, won their Nobel in 2001, Physics Today spelled out how little was known about possible applications for BECs. They are “still on the far horizon,” the publication noted, but listed off possibilities that included playing a role in quantum computing. That’s possible. But Lundblad promises only more experiments, not outcomes.
“I don’t see a pipeline in this work toward practical applications,” Lundblad said. “Certainly on maybe a 20- or 30-year time scale. But it’s not immediate.
“We don’t really understand how these laws of quantum mechanics work for objects that are this big, like millimeter-sized objects. We have a guess and we have a pretty good suspicion, but there’s kind of an open question for how it plays out in practice.”

Creating and manipulating spheres of atoms moving together in a lab in space might sound like a very narrow area of inquiry, he says. “But it actually hits some of the areas in fundamental physics where there are open questions. Like, what happens to well-understood physics systems when you squeeze them to be thin instead of thick? What happens if you curve them around on the surface, rather than having them be flat? What happens if they have holes in the middle instead of being continuous? These are all typical physics questions that we all study in our own way.”
Space represented the next frontier in the exploration of BECs. Creating a BEC on Earth is now a straightforward thing, but continues to be limited by the forces of gravity. The balls of atoms quickly collapse and flatten. Aveline points out that these spherical groupings of atoms created in “traps” in space have much more longevity, opening up more time to watch — and tweak — their behavior. “You can get a lot longer in observation time, or as we call it, interrogation time,” Aveline says.
Lundblad’s proposal to NASA a decade ago was inspired by a physicist from Sussex University in England, Barry Garraway, who in 2001 shared a theory about a way to manipulate the atoms into spheres and other hollow shapes using magnetic fields and radio waves. But in the presence of gravity, Garraway’s trick wouldn’t work.
Lundblad encountered the idea around 2006, when he was doing his postdoctoral work, “playing around with atoms and magnetic fields and radio waves,” he remembers. “And you sort of see that idea and you file it away, like, ‘OK, that’s cool.’” Then he heard about NASA’s call for proposals for the Cold Atom Laboratory in 2013. “I remember someone saying to me like, Hey, do you have any ideas tucked away that really need microgravity?” Lundblad did.
Bringing it full circle, Garraway has now visited Lundblad at Bates twice. “Because I’m a theorist, there is no greater honor than a theorist having their theoretical work starting to be implemented in experimental activity. I mean, that is just so fantastic,” Garraway says. On his second visit to campus, Lundblad had just gotten his first results from the International Space Station. The bubbles looked “a little alien-like,” Garraway says.
Lundblad and Garraway may ultimately publish a paper together. In the meantime, the announcement of the $1.89 million in continued funding on the Cold Atom Laboratory means full steam ahead for Lundblad and his co-investigators Aveline and Smitha Vishveshwara at the University of Illinois Urbana–Champaign, where the grant will support a theoretical physics Ph.D. student.
“The first years were spent laying out some groundwork and techniques and the next step is exploring higher level goals of using that system to study other phenomena,” says Aveline, from a NASA perspective.
Lundblad anticipates that the work he has done and will do on CAL will be his intellectual “meat and potatoes” for more than 10 years. More funding will mean he’ll have the capacity to bring in more students, collaborators, and visiting scientists onto the project (three postdoctoral researchers have worked on the project at Bates thus far). “I want more of that culture here. I want our students to see more scientists, beyond just their faculty.”
In the meantime, Lundblad has praise for deCola, Lindsey, and Veilleux. “They’re fantastic young scientists who’ve all contributed — in very different ways — to pushing the envelope on this research effort.”




